Rifaximin CAS 80621-81-4 API Antibiotics Antibacterial Rifamycinl105sv Red Orange Crystalline Powder
Rifaximin |
CAS No.: 80621-81-4 |
Purity:99% |
Molecular weight: 785.86 g/mol |
Molecular formula: C₄₃H₅₁N₃O₁₁ |
Appearance:Orange-red to reddish-brown crystalline powder |
Package: 1g,10g,100g,1000g |

Rifaximin CAS 80621-81-4 API Antibiotics Antibacterial Rifamycinl105sv Red Orange Crystalline Powder
Color:Dark orange Red orange
Form:Powder
Storage temp:Sealed in dry,Store in freezer, under -20°C
Stability:Hygroscopic
اترك رسالة
ونحن سوف ندعو لكم مرة أخرى في أقرب وقت ممكن !
عزيزي
أنا مهتم في Rifaximin CAS 80621-81-4 API Antibiotics Antibacterial Rifamycinl105sv Red Orange Crystalline Powder ، يمكنك أن ترسل لي المزيد من التفاصيل ، مثل نوع وحجم الحد الأدنى موك ، والمواد ، الخ .
شكراً جزيلاً
في انتظار ردكم .
99% Rifaximin
,Red orange Rifaximin
,80621-81-4
Rifaximin CAS 80621-81-4 API Antibiotics Antibacterial Rifamycinl105sv Red-Orange Crystalline Powder
What is Rifaximin?
Rifaximin is an antibiotic structurally related to rifamycin. It is reported to be efficacious in the treatment of gastrointestinal infections and hepatic encephalopathy, being highly active against Gram-positive and -negative aerobic and anaerobic bacteria. Due to poor systemic absorption, rifaximin is effective in presurgical sterilization of the GI tract.It displays good activity against a wide spectrum of bacteria, including Salmonella spp., S. aureus, and E. coli.
Basic parameters:
| Synonym | rifamycinl105sv |
| MW | 785.89 |
| Melting point | 200-205℃(dec) |
| Storage temp | Sealed in dry,Store in freezer, under -20°C |
| Density | 1.36±0.1 g/cm3(Predicted) |
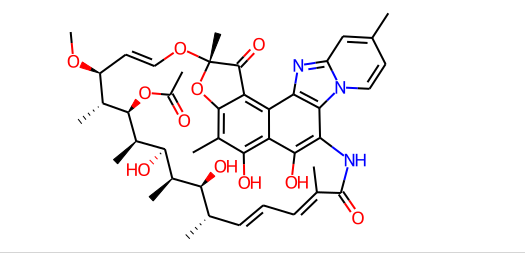
Chemical Formula:
Uses:
Rifaximin is a non-absorbable semisynthetic Rifamycin antibiotic.
Non-absorbable semisynthetic Rifamycin antibiotic
antibacterial, RNA synthesis inhibitor
Pharmacokinetics
Oral absorption is very low. However, a fraction of the dose may be absorbed and rapidly eliminated through the bile. A 400 mg oral dose produces a maximum plasma concentration of 3.8 mg/L after 1.2 h. The plasma half-life is 5.8 h. Up to 90% of the administered dose is concentrated in the gut, less than 0.2% in the liver and kidney, and less than 0.01% in other tissues.
Clinical Use
It is used for a variety of gastrointestinal diseases, including the treatment of traveler’s diarrhea. Preliminary results suggest clinical efficacy in the therapy of hepatic encephalopathy and of C. difficile infections.
Side effects
Oral doses up to 100 mg/kg for 6 months produced no significant signs of toxicity to rats. Teratogenic effects in rats and rabbits have been reported (pregnancy category C).
Very few adverse effects were reported during human treatment, mostly gastrointestinal discomfort. Prolonged therapy was associated with infrequent urticarial skin reactions.
Reference
Chemicalbook http://www.chemicalbook.com
المنتجات الموصى بها











